Biomarker-guided personalized chemotherapy for lung cancer
History
In the treatment of lung cancer, it is important to combine surgery with various drugs and radiation therapy. While surgery is the main treatment for early-stage lung cancer, postoperative adjuvant therapy using anti-cancer drugs can further improve the effectiveness of treatment. Even in advanced lung cancer, if patients can receive effective molecular targeted drugs, immune checkpoint inhibitors, and anti-cancer drug therapy, it becomes possible to carry out multidisciplinary treatment including aggressive surgery.(Reference 8)In this situation, it is important to provide individualized treatment tailored to each individual lung cancer patient.
-
1. The significance of personalized chemotherapy
Lung cancer is a diverse disease with various characteristics, and it is important to provide individualized treatment based on the characteristics of each type of lung cancer.(References 3, 4)This personalized chemotherapy involves "selecting effective anticancer drugs" and "avoiding ineffective anticancer drugs." By using effective anticancer drugs, it is expected that the therapeutic effect will be improved. On the other hand, by avoiding ineffective chemotherapy, it is possible to reduce useless side effects and shorten the useless treatment period. In this way, personalized chemotherapy based on biomarkers will make a great contribution to lung cancer patients.
-
2. Personalized treatment with molecular targeted therapy and immune checkpoint inhibitors
Among molecular targeted drugs, various EGFR tyrosine kinase antagonists, such as osimertinib (trade name Tagrisso), are effective against lung cancer with EGFR gene mutations and are widely used in clinical practice. (Reference 6)Additionally, alectinib (trade name Alecensa) is effective against lung cancer with the ALK fusion gene, and crizotinib (trade name Xalkori) is effective against lung cancer with the ROS1 fusion gene, and are in clinical use. Furthermore, the combination therapy of dabrafenib (trade name Tafinlar) and trametinib (trade name Mekinist) has recently attracted attention for lung cancer with a BRAF gene mutation.
Immune checkpoint inhibitors include nivolumab (trade name Opdivo). Among these, pembrolizumab (trade name Keytruda) is effective against lung cancer with high PD-L1 expression and is in clinical use.
-
3. Current status of clinical practice in Japan
In recent years, many molecularly targeted drugs and immune checkpoint inhibitors have been developed, and when these drugs are effective, treatment outcomes have improved significantly, even for advanced lung cancer. However, lung cancers with EGFR gene mutations account for approximately 40% of adenocarcinomas, lung cancers with ALK fusion genes account for approximately 3% of adenocarcinomas, lung cancers with ROS1 fusion genes and lung cancers with BRAF gene mutations account for approximately 1% of adenocarcinomas each, and lung cancers with high PD-L1 expression account for approximately 20%. Therefore, for the remaining lung cancers and for lung cancers for which molecularly targeted drugs and immune checkpoint inhibitors have become ineffective, further personalized treatment options for anticancer drugs are required.
-
4. thymidylate synthase (TS)
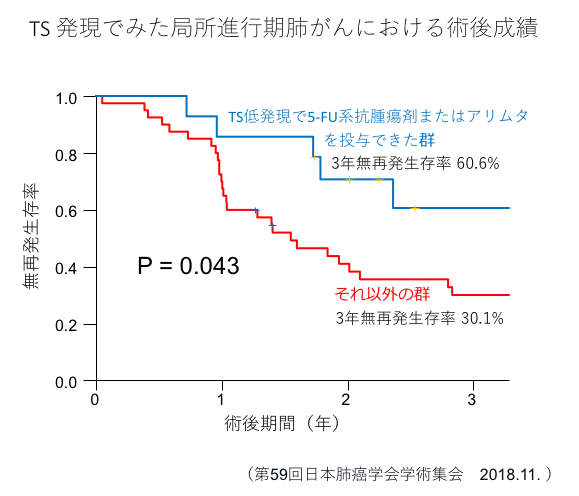
Thymidylate synthase (TS) is a target molecule of 5-FU-based antitumor drugs such as TS-1 and UFT, and we have shown that cancer cells with low TS expression are sensitive to 5-FU-based anticancer drugs.(References 1, 2, 5)In addition, the expression of this TS is also involved in the efficacy of pemetrexed (trade name Alimta) (Br J Cancer 104:1594-601, 2011).
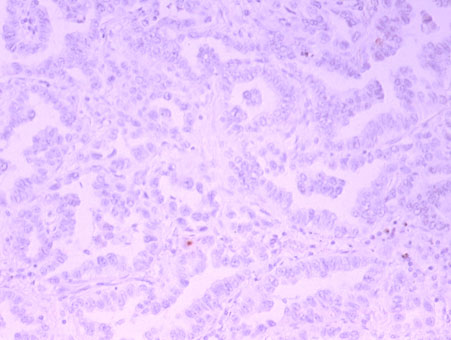
TS low-expressing lung cancer
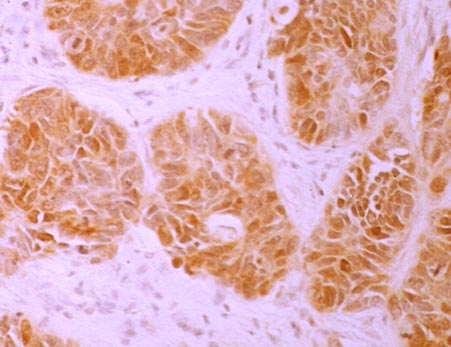
TS-high expressing tumors Lung cancer
-
5. Class III beta-tubulin
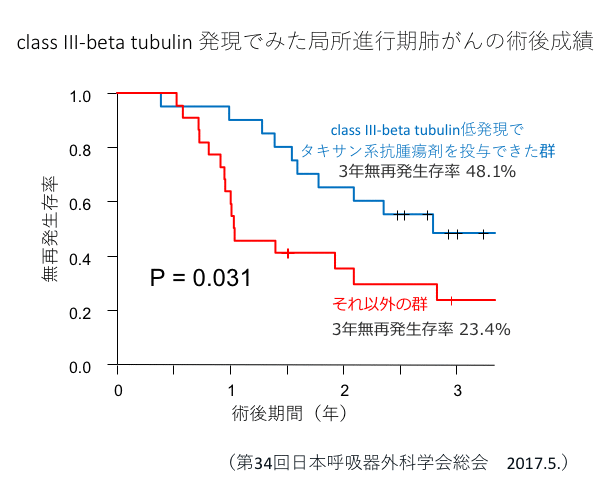
Tubulin is a component of the cytoskeleton. We have shown that the expression of class III beta-tubulin in cancer cells is related to the sensitivity to taxane anticancer drugs such as docetaxel (trade name Taxotere) or paclitaxel (trade name Taxol).(Reference 9).
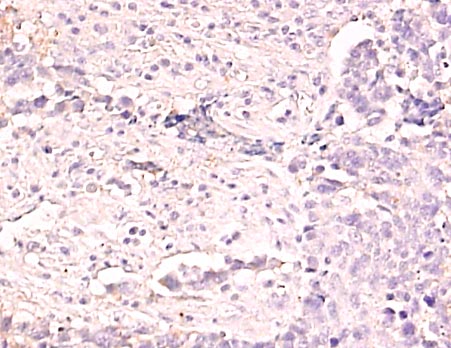
Class III beta tubulin
Low-expressing lung cancer
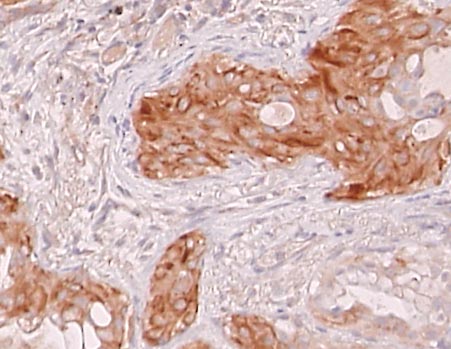
Class III beta tubulin
High-expressing lung cancer
-
Individualized chemotherapy system
February 1, 2006
"Genetic diagnosis of malignant tumors" Approved by a specific approved insurance medical institution Approval number (Takasaki 014) No. 1
November 15, 2011
"Biomarker-based personalized chemotherapy for lung cancer and malignant mediastinal tumors" approved by the Medical Ethics Committee of the Kitano Hospital, Medical Research Institute, Tatsuno Kofukai
February 21, 2012
"Survey on mRNA expression distribution of anticancer drug efficacy predictors in lung cancer" approved by the Medical Ethics Committee of Kitano Hospital, Medical Research Institute, Tatsuno Kofukai
The process of personalized chemotherapy at Kitano Hospital
With sufficient informed consent from patients and their families, we will test for anti-cancer drug-related biomarkers and administer personalized chemotherapy.
Explanation to patients and their families
Before surgery, we explain the details of "biomarker-based personalized chemotherapy" to patients and their families, and obtain their full understanding and consent. During this explanation, we explain that the tests performed for this treatment look at the state of the disease, which is genetic abnormalities in cancer cells, and not the patient's own genetic information.
Tumor tissue extraction and storage
Surgery (through thoracoscopy or thoracotomy), removal of tumor tissue via mediastinoscopy
Testing for antitumor drug-related biomarkers
- Detection of EGFR gene mutations, ALK fusion genes, ROS1 fusion genes, and BRAF gene mutations, and evaluation of PD-L1 expression
- Immunohistochemical evaluation of intratumoral expression: class III beta-tubulin, TS
Biomarker-based personalized therapy
Molecularly targeted drugs
- ・EGFR gene mutation → EGFR tyrosine kinase antagonist administration
- ・ALK fusion gene present → Alecensa administration
- ・ROS1 fusion gene present → Xalkori administration
- ・BRAF gene mutation → Tafinlar + Mekinist
Immune checkpoint inhibitors
- ・High PD-L1 expression → Keytruda administration
antitumor agents
- ・Low TS expression → Administration of 5FU anticancer drugs or Alimta
- ・Low expression of class III beta-tubulin → Administration of taxane anticancer drugs
Treatment results
Press
- The latest treatment for lung cancer. Setouchi TV Next Challenge to the Future File 90 (2005)
- Respiratory system: Custom-made anti-cancer drug treatment. TV Setouchi Health Encyclopedia (November 10, 2005)
Literature (our main research papers)
- Huang C, Yokomise H, Kobayashi S, Fukushima M, Hitomi S, Wada H. Intratumoral expression of thymidylate synthase and dihydropyrimidine dehydrogenase in non-small cell lung cancer patients treated with 5-FU-based chemotherapy. International Journal of Oncology 17: 47-54, 2000.
- Huang C, Liu D, Masuya D, Nakashima T, Kameyama K, Ishikawa S, Ueno M, Haba R, Yokomise H. Clinical application of biological markers for treatments of resectable non-small cell lung cancers. British Journal of Cancer 92: 1231-1239, 2005.
- Huang C, Yokomise H, Fukushima M, Kinoshita M. Tailor-made chemotherapy for non-small cell lung cancer patients. Future Oncology 2: 289-299, 2006.
- Huang C, Yokomise H, Liu D. Therapeutic strategy based on biological markers for non-small cell lung cancers. Enhancer-Biotherapy Cancer 4: 19-21, 2006.
- Nakano J, Huang C, Liu D, Masuya D, Nakashima T, Yokomise H, Ueno M, Wada H, Fukushima M. Evaluations of biomarkers associated with 5-FU sensitivity for non-small cell lung cancer patients postoperatively treated with UFT. British Journal of Cancer 95: 607-615, 2006.
- Liu D, Nakano J, Ueno M, Masuya D, Nakashima T, Yokomise H, Yube K, Huang CA useful protocol for analyzes of mutations of the epidermal growth factor receptor gene. Oncology Reports 15: 1503-1505, 2006.
- Huang C, Liu D, Nakano J, Yokomise H, Ueno M, Kadota K, Wada H. E2F1 overexpression correlates with thymidylate synthase and survivin gene expressions and tumor proliferation in non-small cell lung cancer. Clinical Cancer Research 13: 6938-6946, 2007.
- Yokomise H, Gotoh M, Okamoto T, Yamamoto Y, Ishikawa S, Nakashima T, Masuya D, Liu D, Huang CInduction chemoradiotherapy (Carboplatin-taxanes and concurrent 50 Gy radiation for bulky c-N2, N3 non-small-cell lung cancer. Journal of Thoracic and Cardiovascular Surgery 133: 1179-1185, 2007.
- Huang C, Kadota K, Liu D, Ueno M, Nakashima N, Ishikawa S, Gotoh M, Misaki N, Chang S, Yokomise H. Expression of ERCC1 and class III beta-tubulin is associated with the survival of resected stage III non-small cell lung cancer patients treated with induction chemoradiotherapy using carboplatin-taxane. Experimental and Therapeutic Medicine 1: 445-451, 2010.
Conference presentations
- Hwang, Zhenglong. Biomarker-based personalized treatment for non-small cell lung cancer. Luncheon seminar at the 83rd Kinki Regional Meeting of the Japanese Respiratory Society. June 28, 2014, Himeji.
- Hirai T, Shoji T, Sumitomo R, Huang Zhenglong. Evaluation of class III beta-tubulin expression for personalized chemotherapy in non-small cell lung cancer. 73rd Annual Meeting of the Japanese Cancer Association. September 25, 2014. Yokohama.
- Huang Zhenglong, Liu Dage, Yokomise Yuyasu, Wada Hiromi. Personalized treatment of lung cancer using thymidylate synthase and development of nucleic acid medicine. The 32nd Annual Meeting of the Japanese Society for Thoracic Surgery. May 15, 2015. Takamatsu.
- Sumitomo R, Hirai T, Huang Zhenglong. Relationship between intratumoral expression of TS and RRM1 and tumor proliferation in non-small cell lung cancer. 75th Annual Meeting of the Japanese Cancer Association. October 6, 2016. Yokohama.
- Sumitomo R, Fukui T, Otake Y, Huang Zhenglong. The usefulness of intratumoral class III beta-tubulin expression in postoperative adjuvant chemotherapy for advanced non-small cell lung cancer. 34th Annual Meeting of the Japanese Society for Thoracic Surgery. May 18, 2017. Fukuoka.
- Sumitomo R, Hirai T, Murakami Y, Otake Y, Huang Zhenglong. Usefulness of intratumoral thymidylate synthase expression in postoperative adjuvant chemotherapy for advanced non-small cell lung cancer. 59th Annual Meeting of the Japan Lung Cancer Society. November 29, 2018. Tokyo.






