
Departments
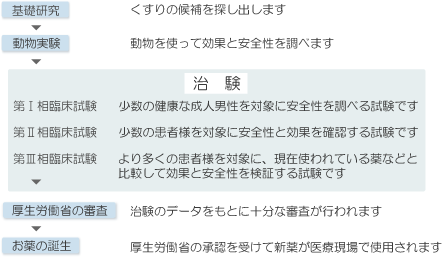
Before a new drug can be used, it is necessary to conduct tests (clinical trials) with the cooperation of many patients to verify the efficacy and safety of the candidate drug.
Only after the efficacy and safety of a drug candidate have been confirmed and approved by the government (Ministry of Health, Labour and Welfare) can it be used as a medicine.
Clinical trials such as these, conducted using candidate drugs to obtain approval from the government (Ministry of Health, Labour and Welfare), are called "clinical trials."
Clinical trials are conducted under strict rules, such as the "Ministerial Ordinance on Good Clinical Practice (GCP)" established by the Ministry of Health, Labour and Welfare, in order to protect the human rights and safety of participants.
Clinical Research Coordinators (CRCs) are specialized staff who provide various support to patients and doctors to ensure that clinical trials are conducted smoothly. In addition to explaining clinical trials to patients in an easy-to-understand manner, they also accompany patients to medical examinations and tests.
If you have any questions or concerns about the clinical trial, please feel free to contact us at any time.
If you are interested in participating in a clinical trial, please talk to your doctor.
The Institutional Review Board reviews the appropriateness of conducting a clinical trial from ethical, scientific, medical and pharmaceutical perspectives.
Clinical trials conducted at our hospital are reviewed and approved by the Institutional Review Board established within our hospital to determine whether it is appropriate to conduct the clinical trial at our hospital.In addition, ongoing clinical trials are reviewed and reviewed as necessary to determine whether they should continue.
The IRB's procedures and committee members are published below. These procedures are established in accordance with the Ministerial Ordinance on Good Clinical Practice (GCP) and related notifications, and clinical trials at our hospital are conducted in accordance with these procedures.
We have made public the summary of the minutes of the Institutional Review Board meetings held at our hospital. If you would like to view the summary of the minutes of the Institutional Review Board meetings held before fiscal year 2014, please contact us at the address below.
From the perspective of intellectual property rights, some items have been masked at the request of the clinical trial sponsor or the person conducting the clinical trial.
Kitano Medical Research Institute, Public Interest Incorporated Foundation, Tatsunokofukai
Clinical Trial Consultation Office
TEL: 06-6131-2839