Medical Research Institute Overview
Working with Kyoto University for the advancement of medicine
It was founded in 1925.Tazuke KofukaiResearch Institutehas contributed to the advancement of medicine through both clinical research and basic research based on medical care at Kitano Hospital. The results of this research are now being applied to cutting-edge medical treatments, such as spinal cord regeneration and eardrum regeneration. These research efforts have been supported for many years by close personnel exchanges with Kyoto University and collaboration in a wide range of fields. Going forward, each and every staff member will continue to contribute to the advancement of medicine, not only as a medical professional, but also without forgetting to consider the perspective of a scientist.
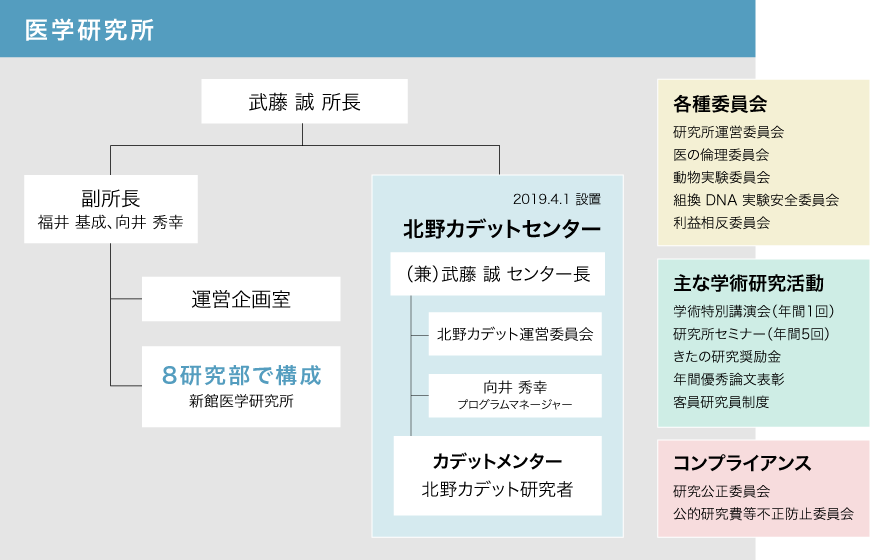
Our institute consists of eight research departments, the Kitano Cadet Center, and the Management and Planning Office. The greatest strength of our institute is that we are affiliated with Kitano Hospital, which boasts one of the best staffing levels in Osaka. We have close ties with each department and division at Kitano Hospital, and a large number of clinical studies are being conducted based on an extremely large number of clinical cases.
In terms of basic research, animal experiments and genetic research are conducted day and night, primarily in the new research wing, in order to verify and develop new ideas and hypotheses derived from clinical experience. Professor Emeritus Takeshi Watanabe of Kyushu University has been appointed as a special research advisor, and we are also committed to nurturing young researchers. We are also fully equipped with the equipment essential for research, such as real-time PCR, flow cytometers, Bioplex, confocal microscopes, and virtual slide devices.
Research Institute
Since fiscal year 2016, our institute has been reorganizing its organizational structure and building a support system that allows researchers to devote themselves to their research. The Medical Research Institute is responsible for the following tasks:
- Clinical study design, support for statistical protocol creation, monitoring and auditing of clinical studies
- Research data management, allocation work, etc.
- Supervision and work control of research assistants who assist researchers in their research
- Coordination of clinical research
- General research and administrative work related to public research funds, planning and research related to the research institute, and reporting fraud
- Kitano Cadet
Research support for the Institute
- Establishment of a support system for the new research institute
Young researchers aiming to conduct basic research can receive guidance on specific methods for conducting their research from Special Research Advisor Takeshi Watanabe.In addition, research assistants are actively involved in supporting researchers who conduct research in between their busy clinical work.Furthermore, a full-time research institute manager is in charge of maintaining the environment in the new research wing.
- Support system for obtaining scientific research grants
In fiscal year 2015, our institute's staff served as representatives for 14 Grant-in-Aid for Scientific Research projects. We also have two joint research projects with other universities. A dedicated administrative staff member supports administrative tasks such as application and management.
- Kita Research Grants, etc.
We provide research funding support to approximately three outstanding research projects once a year. We also award an annual Outstanding Paper Award, provide assistance with paper submissions, and subsidize travel expenses for overseas conference presentations.
- Support for research planning and statistical analysis
Research advisor Toshiro Katayama provides consultations regarding research design and statistical analysis at the Medical Research Support Center's Quality Control Office.
- Establishment of an ultra-low temperature specimen storage system
We have a system in operation that centrally manages valuable clinical specimens in the laboratory's ultra-low temperature freezer. It can also be linked to databases such as electronic medical records. In the future, we would like to begin considering ultra-low temperature storage of tissues and other materials.
- Building a clinical research database
By integrating and sharing the various clinical research data generated by each research department, we hope to support the creation of clinical research from new perspectives.
For the advancement of medicine, we take a strict stance against research misconduct.
The Institute recognizes that "misconduct in research activities goes against the very essence of science, which is the pursuit of truth and the creation of new knowledge; it undermines people's trust in science, hinders its development, and is a desecration that cannot be tolerated," and has established the following regulations and will take a strict stance against such misconduct.
Promoting fair research activities
Related Links
- Science Council of JapanCode of Conduct for Scientists - Revised EditionJanuary 25, 2013
- Japan Society for the Promotion of ScienceFor the Sound Development of Science: The Attitude of an Honest Scientist" March 2015
- Ministry of Education, Culture, Sports, Science and TechnologyGuidelines for Responding to Misconduct in Research Activities(Decided by the Minister of Education, Culture, Sports, Science and Technology on August 26, 2014)
- Ministry of Health, Labour and Welfare"Guidelines for Responding to Misconduct in Research Activities in the Field of Health, Labor and Welfare(decided on January 16, 2015)
Appropriate management of public research funds
Consultation and reporting desk regarding public research funds, etc.
Tazuke Kofukai Medical Research Institute, a public interest incorporated foundation
Medical Research Institute Management Planning Office
Request for support for research activities for the advancement of medicine
Requests regarding specimen storage and use
At our hospital, we may retain some of the blood, urine, biopsy tissue, and other samples collected for clinical testing and diagnosis, and use them for research, education, etc., while strictly adhering to professional and legal confidentiality obligations (personal information, test results, etc.).
When research results are presented at academic conferences or in paper form, each individual will be kept anonymous and personal information will not be made public.
If we wish to use the samples used in the tests for genome/gene analysis research, we must first obtain review and approval from the Medical Ethics Committee, which is composed of the hospital's vice-director, relevant department heads, and lawyers, as well as permission from the hospital director.
If you do not agree with these points, please feel free to tell your doctor.

